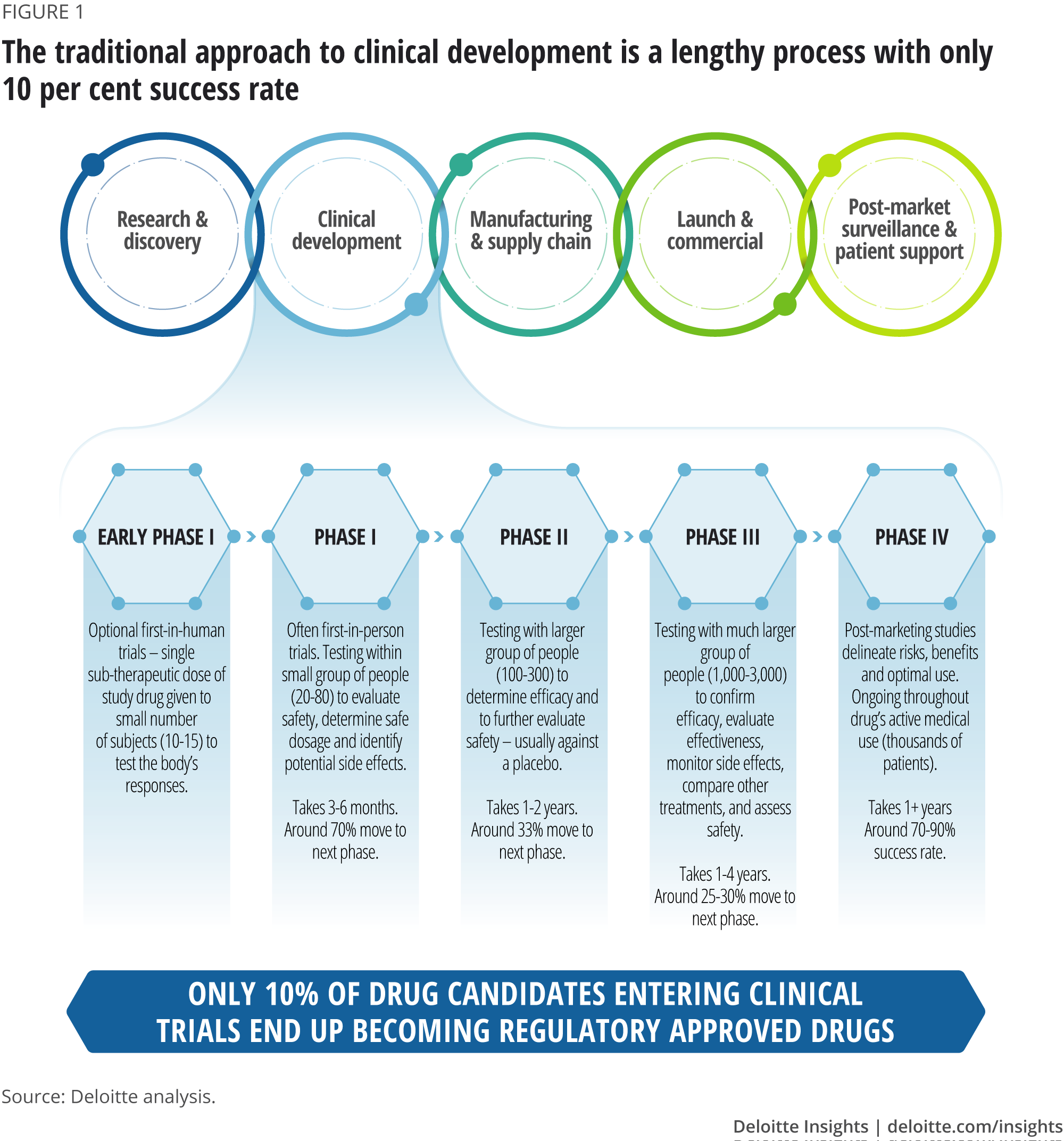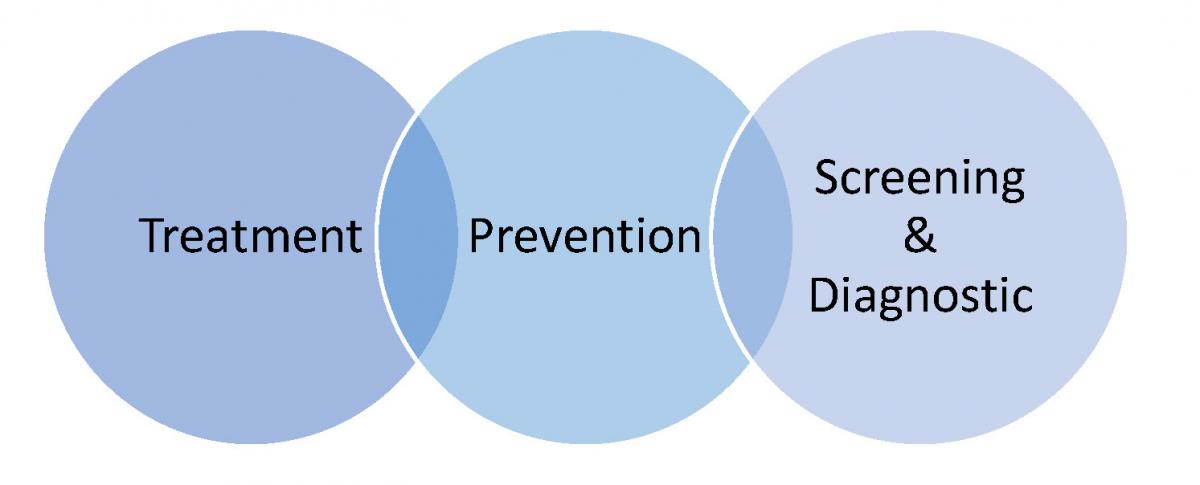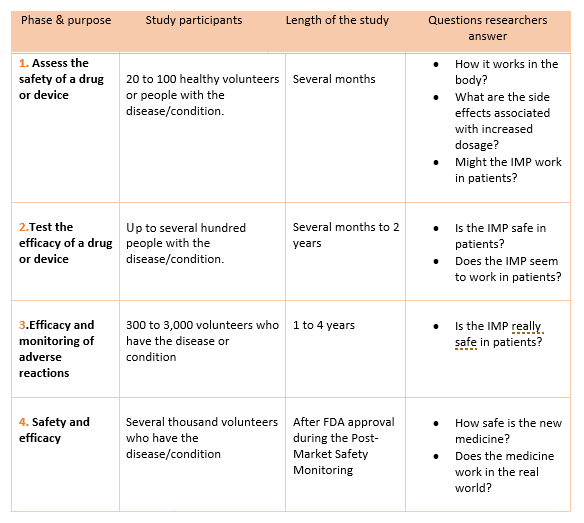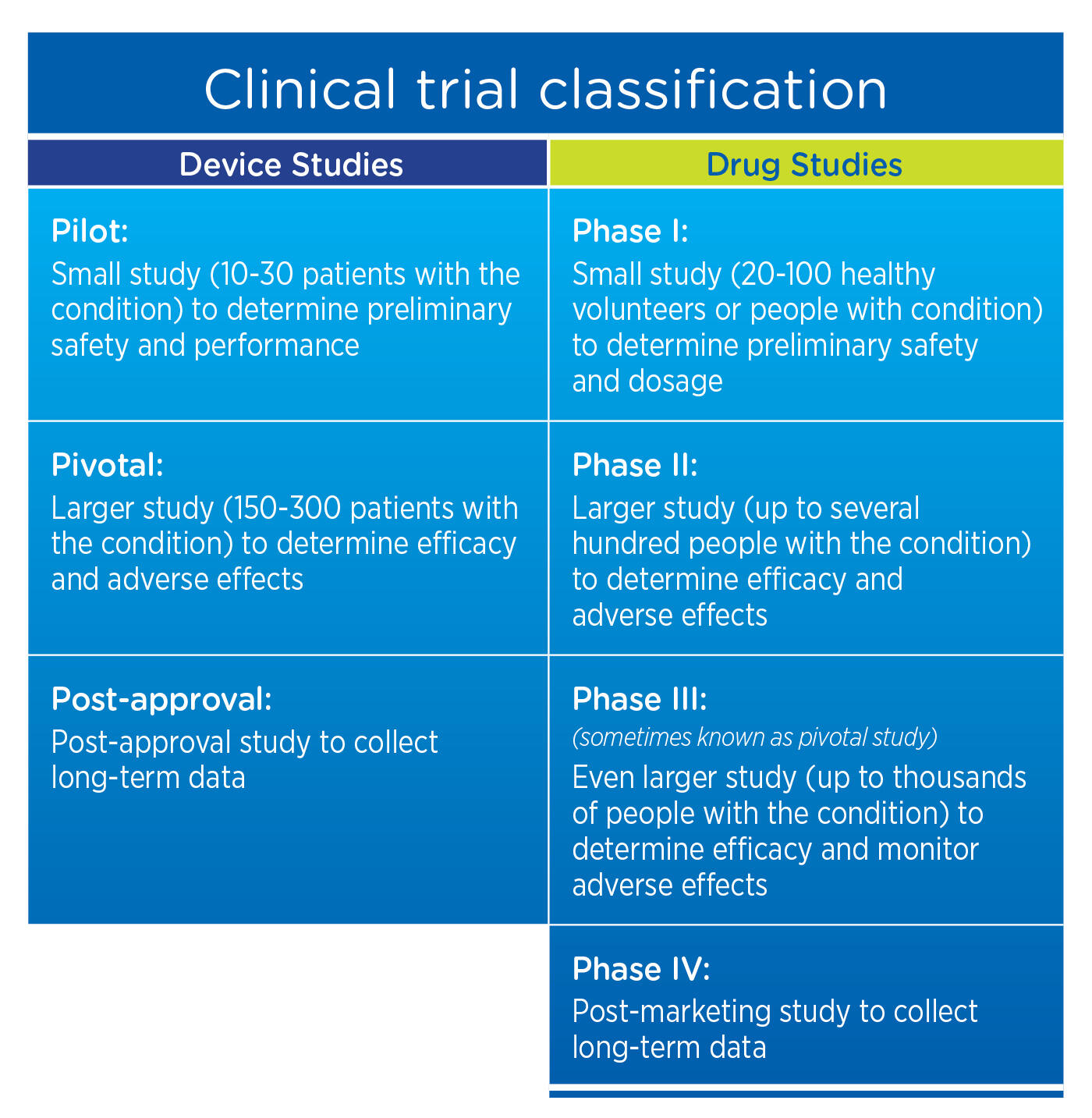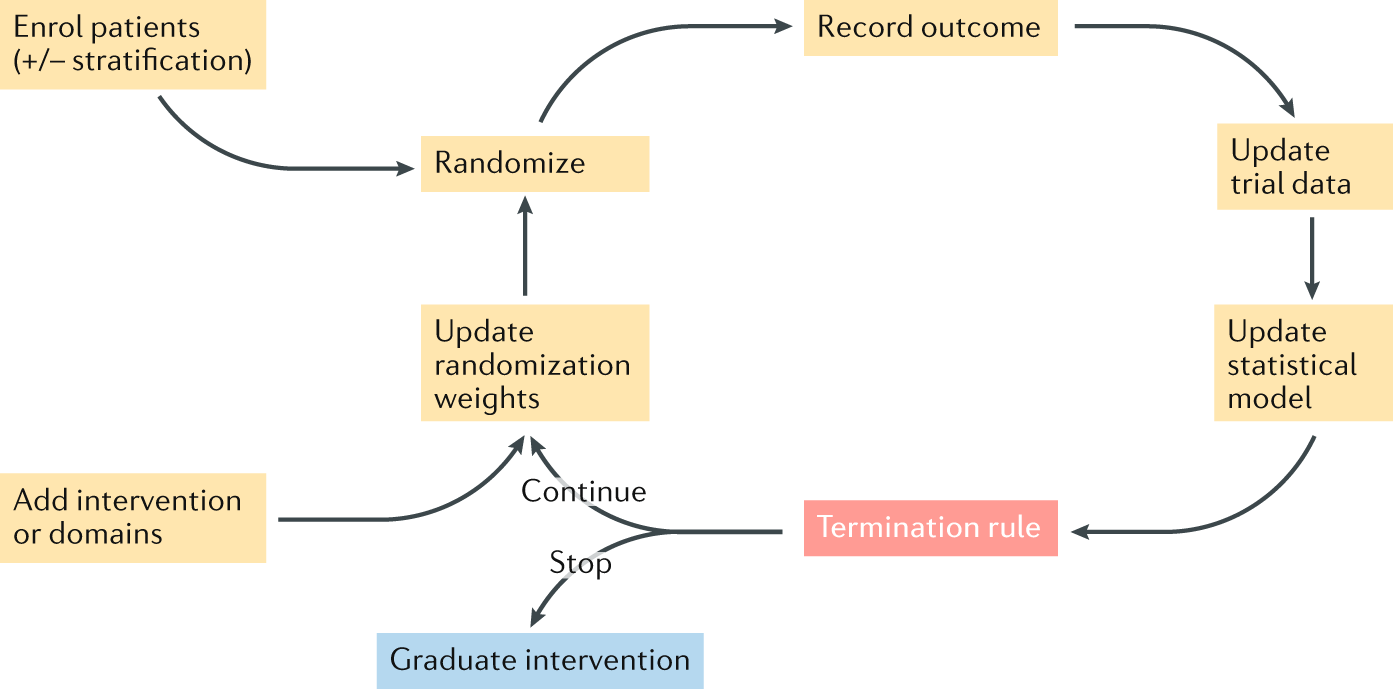
Adaptive platform trials: definition, design, conduct and reporting considerations | Nature Reviews Drug Discovery

Decentralized clinical trials. A framework for “direct-to-patient,”… | by Andy Coravos | HumanFirst | Medium

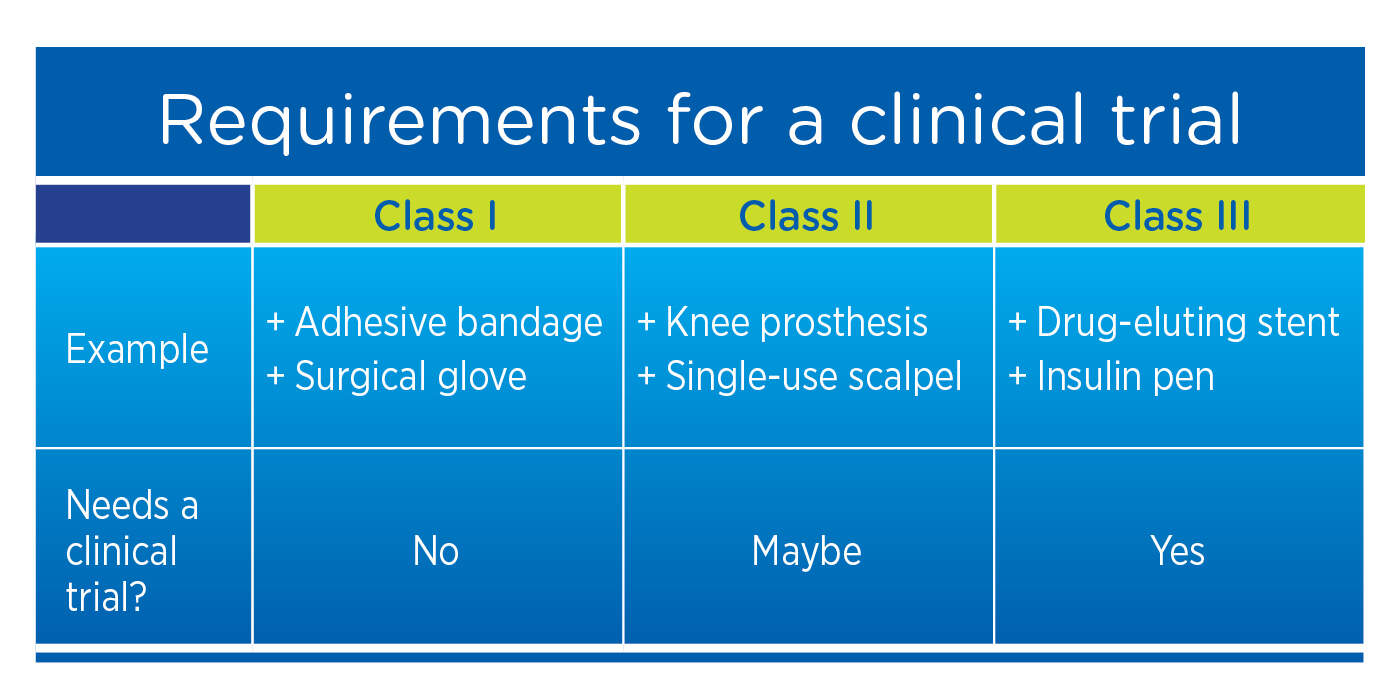
Draft Guidance Document: Applications for Medical Device Investigational Testing Authorizations - Canada.ca
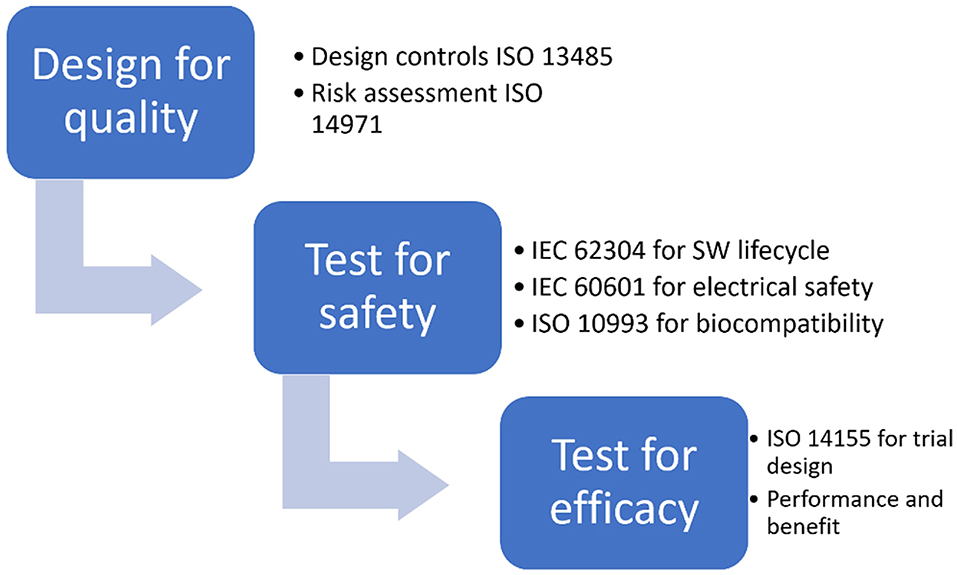
Frontiers | Comprehensive Review on Current and Future Regulatory Requirements on Wearable Sensors in Preclinical and Clinical Testing | Bioengineering and Biotechnology

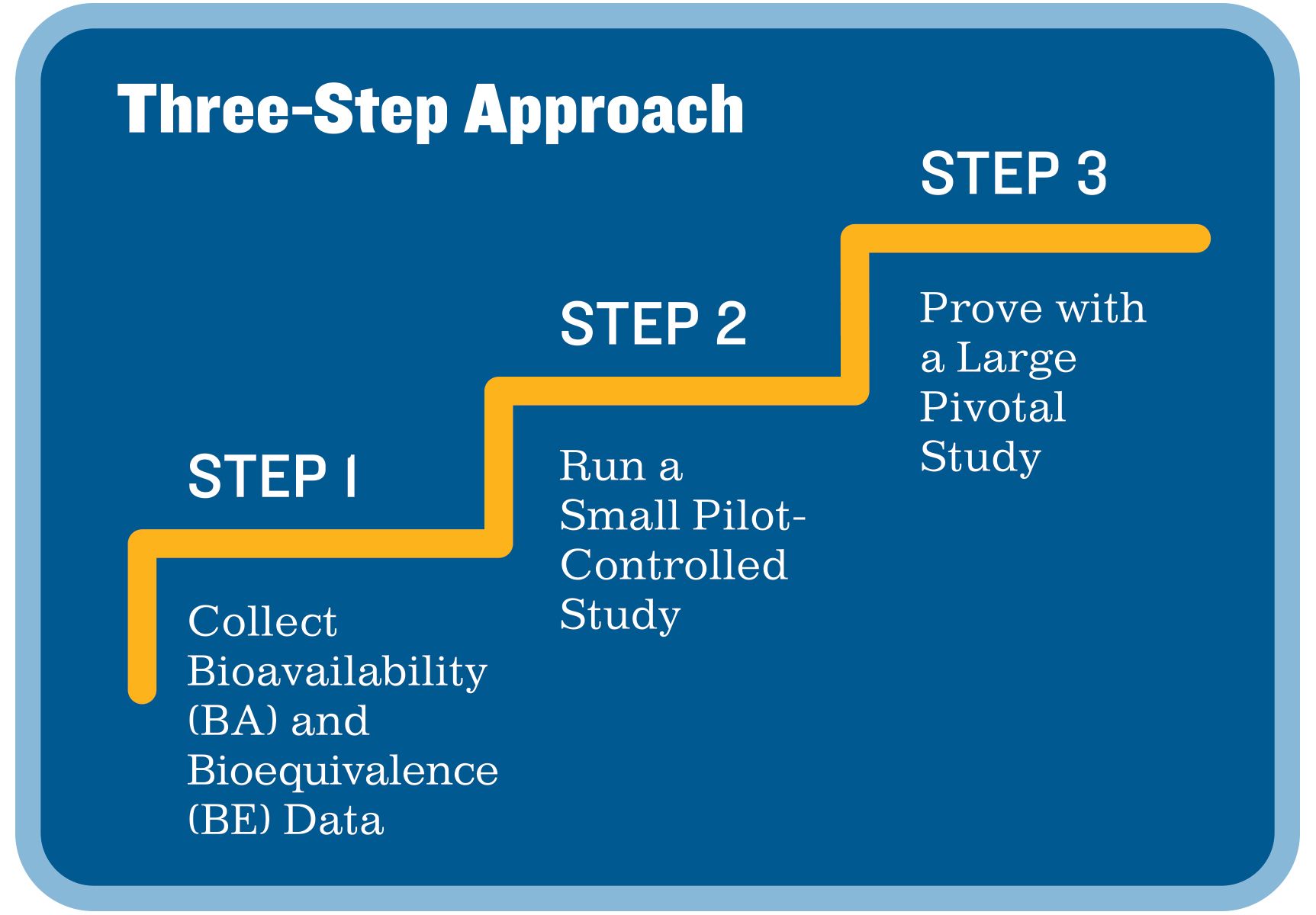
Pivotal Clinical Study to Evaluate the Safety and Effectiveness of the MANTA Percutaneous Vascular Closure Device | Circulation: Cardiovascular Interventions